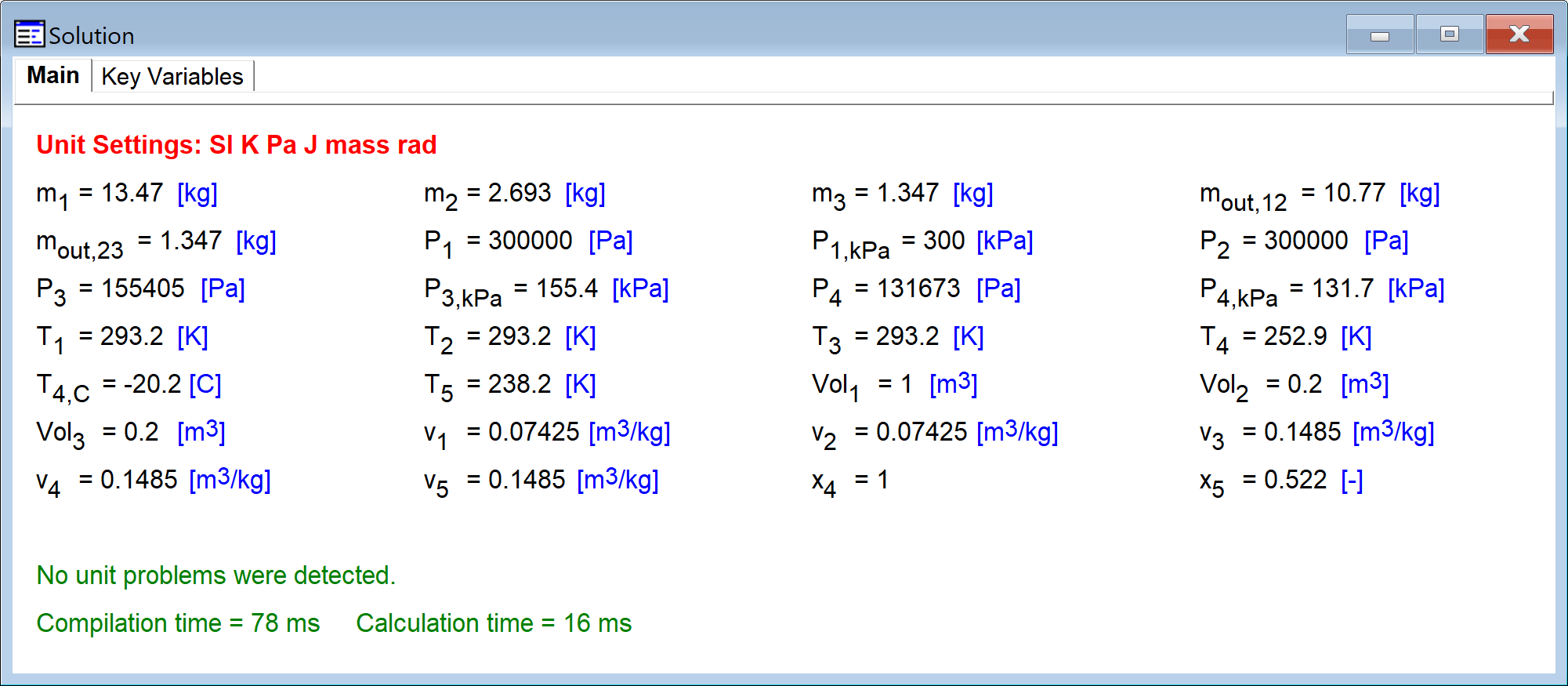
Sample Problem Solution
You need to develop a solution to the problem in EES that uses the variable names and units called out in the problem statement. My solution is below.
$UnitSystem SI Mass J K Pa
$TabStops 4 in
"Inputs"
P_1_kPa = 300 [kPa]
Vol_1 = 1 [m^3]
"Part a: what is the mass, m_1 (kg)"
P_1=P_1_kPa*Convert(kPa,Pa) "initial pressure"
T_1=ConvertTemp(C,K,20 [C]) "initial temperature"
v_1=Volume(R134a,T=T_1,P=P_1) "specific volume"
m_1=Vol_1/v_1 "initial mass"
"Part b: how much mass is removed, m_out_12 (kg)"
Vol_2=Vol_1*0.2 "volume at state 2"
P_2=P_1 "pressure at state 2"
T_2=ConvertTemp(C,K,20 [C]) "temperature at state 2"
v_2=Volume(R134a,T=T_2,P=P_2) "specific volume"
m_2=Vol_2/v_2 "mass at state 2"
0=m_out_12+m_2-m_1 "mass balance, m_out_12 is the mass extracted during process 1 to 2"
"Part c: pressure after 50% of the remaining mass is removed, P_3_kPa (kPa)"
m_out_23=0.5*m_2 "half of the remaining mass is extracted"
0=m_out_23+m_3-m_2 "mass balance"
Vol_3=Vol_2
T_3=ConvertTemp(C,K,20[C]) "temperature"
v_3=Vol_3/m_3 "specific volume"
P_3=Pressure(R134a,T=T_3,v=v_3) "pressure"
P_3_kPa = P_3*Convert(Pa,kPa) "pressure in kPa"
"Part d: temperature when condensation begins T_4_C (C)"
"Part e: pressure when condensation begins P_4_kPa (kPa)"
v_4=v_3 "specific volume"
x_4=1 "quality"
P_4=Pressure(R134a,x=x_4,v=v_4) "pressure"
P_4_kPa = P_4*Convert(Pa,kPa) "pressure in kPa"
T_4=Temperature(R134a,x=x_4,v=v_4) "temperature"
T_4_C = ConvertTemp(K,C,T_4) "temperature in C"
"Part f: quality when temperature reaches -35 C"
T_5 = ConvertTemp(C,K,-35 [C]) "temperature"
v_5 = v_4 "specific volume"
x_5 = Quality(R134a,T=T_5,v=v_5) "quality"
The solution is shown below.
