Contents
- Index
Entropy departure is the dimensionless value (s[ideal]-s)/(R). s[ideal]-s is the difference in entropy between an ideal gas and a real gas at the same temperature and pressure. R is the gas constant. The negative of the entropy departure for the Redlich-Kwong-Soave equation of state is:
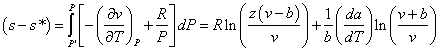
Example:
Tr=1.2
Pr=1.5
omega=0.1
ds=EntrDep(Tr, Pr, omega)
{Solution: ds=0.9211}