Contents - Index - Previous - Next
H_DEP_PR
H_DEP_PR (Tr, w, Z, B) returns the dimensionless enthalpy departure of a pure fluid according to the Peng-Robinson equation of state
where
Tr is reduced temperature
w is the acentric factor
Z is the compressibility factor
B is the second Peng-Robinson equation of state parameter
The dimensionless enthalpy departure is the difference in enthalpy between an ideal gas (designated with a superscript 0) and a real gas at the same temperature and pressure divided by (R Tc) where R is the gas constant and Tc is the critical temperature.
![]()
Note that Z is provided by functions Z_G_PR and Z_L_PR and B is provided by procedure AB_PR for pure fluids.
The enthalpy departure of a mixture cannot be determined with this function since Tr for a mixture is not defined. Using the Peng-Robinson equation for a mixture, the enthalpy departure for a mixture can be expressed:
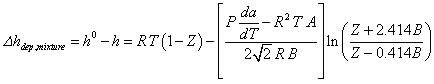
Procedure AB_MIX_PR can be used to determine values of A and B for the mixture and functions Z_G_PR and Z_L_PR provide the value of Z for the mixture. The value of da/dT for the mixture is provided by function DSADT_MIX_PR.
Example:
Tr=0.85
Pr=0.5
omega=0.05
Call AB_PR(Tr, Pr, omega: A,B)
Z_G=Z_G_PR(A,B)
R=R#
Tc=190.6 [K] "methane"
DELTAh=H_DEP_PR(Tr,omega,Z_G,B)*R*Tc
{Solution:
A=0.3391
B=0.04576
DELTAh=1608 [kJ/kmol]
omega=0.05
Pr=0.5
R=8.314 [kJ/kmol-K]
Tc=190.6 [K]
Tr=0.85
Z_G=0.5748
}