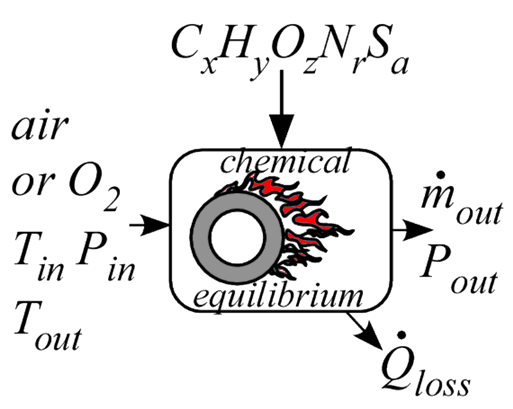
Combustor4_CL

Procedure Combustor4_CL provides a model of a combustor is similar to Combustor3_CL with the major difference being that it accepts the outlet equilibrium temperature and calculates the heat transfer rate to the combustion chamber. As with Combustor3_CL, this component accepts the name of the fuel, which can be a name contained in the list of ideal and NASA gases or any chemical formula consisting of elements C, H, O, N, and S. The procedure assumes complete combustion with dry air or pure oxygen. No fuel or fuel fragments remain. The percentage of excess air (or oxygen) above the stoichiometric requirement is provided in input Excess%. At the elevated temperatures occurring in a combustion process, dissociation reactions that produce CO, NO, OH and other species can occur to a significant extent. This model is specifically designed to consider dissociation reactions. The product gas is assumed to be a mixture of CO2, CO, H2O, H, H2, OH, O, O2 ,NO, NO2, N2, N, SO2 and SO3 and the equilibrium mole fractions of these gases are determined in the analysis.
The heat transfer rate from the combustion vessel is an output of this component. The fuel is assumed to be supplied at the same temperature as the air. A negative heat transfer rate indicates energy needs to be transferred to the equilibrium gas mixture to achieve the specified outlet temperature.
Inputs:
F$: Name of a fuel or fuel mixture consisting of C, H, O, N and S, e.g., C4H10 or 0.95*CH4+0.05*C2H6. Natural_gas and Natural_gas2 are also accepted.
LHV: average lower heating value of the fuel (J/kg, kJ/kg, Btu/lb_m) (set to 0 if F$ is an EES substance)
T_in: inlet temperature of air and fuel (K, C, F, or R)
P_in: inlet pressure of air (bar, atm, Pa, kPa, MPa)
OX$: string constant or string variable that is set to either 'Air' or 'O2'
m_dot_fuel: inlet mass flow rate of fuel (kg/s or lb_m/hr) {note that this differs from Combustor2_CL}
Excess%: Percent excess air - 0 is stoichiometric. Must be >=0
DPoverP: pressure loss in combustor normalized by inlet pressure
T_out: outlet temperature (K, C, F, or R)
Outputs:
m_dot_out: outlet mass flow rate of combustion gases(kg/s or lb_m/hr)
P_out: outlet pressure (bar, atm, Pa, kPa, MPa)
Q_dot_loss: rate of heat transfer from the combustion vessel to surrounding (W, kW, or Btu/hr)
AF: ratio of the mass flow rate of air (or oxygen) to the mass flow rate of fuel
x[1..14] mole fractions of CO2, CO, H2O, H, H2, OH, O ,O2 ,NO, NO2 ,N2, N, SO2, SO3
C$[1..14] names of the gases in the equilibrium mixture
Only the first three outputs are required.
Note: F$ identifies the composition of the fuel. F$ can be set to any recognized EES substance in the built-in ideal gas or NASA library provided the substance is composed of elements C, H, O, N and S. In addition, a mixture can be specified by using the + symbol with each species multiplied by its mole fraction. For example to specify a mixture of 95% CH4 and 5% CO2, set F$ to 0.95*CH4+0.05*CO2. However, if F$ is not a recognized fluid name, it is necessary to provide the lower heating value of the fuel per unit MASS of the fuel mixture.
Example: {Chemical Equilibrium of a Diesel fuel}
$Load Component Library
$UnitSystem SI C kPa mass kJ
$VarInfo P_out Units=kPa
$VarInfo T_out Units=C
$VarInfo MW Units=kg/kmol
$VarInfo m_dot_out Units=kg/s
$VarInfo Q_dot_loss Units=kW
T_in=25 [C]
P_in=101.3 [kPa]
m_dot_fuel=0.0005 [kg/s] "mass flow rate of fuel"
Excess%=50 [%]
DPoverP=0.0
T_out=1850 [C]
F$='C12H23S0.1' {note that the fuel includes some sulfur}
LHV=45000 [kJ/kg] {specify 0 so EES uses the known LHV for this fluid}
OX$='air'
CALL Combustor4_CL(F$, LHV, T_in, P_in, OX$, m_dot_fuel, Excess%, DPoverP, T_out: m_dot_out, P_out, Q_dot_loss, AF, MW, x[1..14], C$[1..14])
\
{Solution:
AF=17.37
DPoverP=0
Excess%=50 [%]
F$='C12H23S0.1'
LHV=45000 [kJ/kg]
MW=30.22 [kg/kmol]
m_dot_fuel=0.0005 [kg/s]
m_dot_out=0.009185 [kg/s]
OX$='air'
P_in=101.3 [kPa]
P_out=101.3 [kPa]
Q_dot_loss=1.301 [kW]
T_in=25 [C]
T_out=1850 [C]
CO2 0.1307
CO 0.0007113
H2O 0.01547
H 0.00001473
H2 0.00001677
OH 0.001885
O 0.001042
O2 0.06518
NO 0.005835
NO2 0.00001166
N2 0.778
N 1.697E-09
SO2 0.001093
SO3 0.000002825
}
See also: Combustor1