NASA Ideal Gas and Condensed Species Data
Thermodynamic property data for 1262 ideal gases and 762 condensed materials are available in the EES NASA property data base.
These data were compiled by NASA as documented in:
Bonnie J. McBride, Michael J. Zehe, and Sanford Gordon
"NASA Glenn Coefficients for Calculating Thermodynamic Properties of Individual Species"
NASA/TP-2002-211556, Sept. 2002
https://www.grc.nasa.gov/WWW/CEAWeb/
The data consist of the molar mass, specific heat, specific enthalpy, and specific entropy at standard pressure as a function of temperature. The specific enthalpy is referenced to the elements having zero enthalpy at 25 C. The specific entropy is referenced to the pure fluid having zero entropy at 0 K, 1.013 bar, in accordance with the 3rd Law of Thermodynamics. (Pressure is not used for the condensed substances).
It is necessary to load the NASA library before it can be used. Starting with version 11.586, an option is provided in the Auto Load Libaries tab of the Preferences dialog to automatically load the NASA Substances library when EES is started. By default, this library is not automatically loaded. Loading the library requires little computer time, but compilation time for large EES programs may be slower when the library is loaded. If you chose to not automatically load the NASA Substances at startup, you can manually load it by placing a $Load NASA directive at the top of the Equations window, as shown in the example. If the library is already loaded, this directive is ignored.
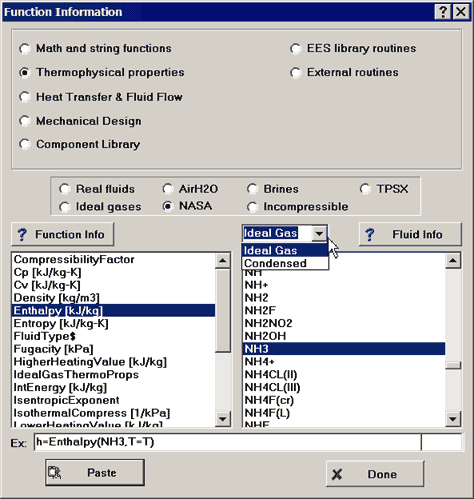
The list of substances and available substances is most easily accessed from the Function Information dialog. Select the Thermophysical properties button and then the NASA button. A drop-down control will appear to allow a choice of ideal gas or condensed substances.

The NASA ideal gas and condensed data can then be used in EES property functions in exactly the same manner as for the built-in ideal gases. The unit system for which properties such as temperature and pressure must be supplied and properties are returned is specified with the Unit System dialog or the $UnitSystem directive. The thermal properties are often most useful when expressed on a molar basis, which can be set with the choice of the unit system. Examples uses of the NASA substances with the EES property routines are:
$Load NASA
$UnitSystem SI K Pa molar
h_NH3=enthalpy(NH3,T=500 [K]) {molar specific enthalpy of ammonia ideal gas at 500 K}
s_H=entropy(H,T=2000 [K],P=Po#) {molar specific entropy of ammonia ideal gas at 2000 K and atmospheric pressure}
c_carbon=cp(C(GR),T=298.15 [K]) {molar specific heat of solid carbon}
s_carbon=entropy(C(GR),T=500 [K]) {specific entropy of solid carbon at 500 K}
MW_AL=molarmass(AL(CR)) {molar mass of aluminum}
{Solution:
c_carbon=8.527 [kJ/kmol-K]
h_NH3=-38126 [kJ/kmol]
MW_AL=26.98 [kg/kmol]
s_carbon=11.66 [kJ/kmol-K]
s_H=154.3 [kJ/kmol-K]}
One major difference between the built-in EES and NASA property data is that transport properties (viscosity and thermal conductivity) are not available. Also, critical properties and other related data, such as the acentric factor, triple point, heat of fusion, etc. are not available for the NASA data.
The enthalpy of incompressible substances should be a function of both temperature and pressure. However, density data are not available for the NASA condensed substances so the effect of pressure on the specific enthalpy cannot be determined. It should be understood that the specific enthalpy provided for NASA condensed substances is applicable for low (e.g., atmospheric) pressure.
The NASA data were originally provided in the NASA external procedure. This external procedure is still provided for backward compatibility but it requires temperatures in K and all properties are returned on a molar basis in the SI system with kJ as the energy unit, regardless of the EES unit system settings. As an example, the following call provides thermodynamic property data for pure solid carbon.
Call NASA('C(gr)',300 [K]: cp, h, s) {returns property information for solid carbon on a molar basis in SI units}
The NASA_EES procedure provides the same capability but it uses the unit system specified with the Unit System dialog or the $UnitSystem directive. As an example, the following Call statement will determine the properties of solid carbon in English units.
$Load NASA
$UnitSystem Eng F psia molar
T=77 [F]
Call NASA_EES('C(gr)',T: cp, h, s)
{Solution
cp=2.037 [Btu/lbmol-R]
h=0.000 [Btu/lbmol]
s=1.369 [Btu/lbmol-R]
T=77 [F]}
The NASA library can be unloaded with the $UNLOAD NASA directive. It can be loaded at startup with the options on the Auto Load Libraries page in the Preferences dialog.